
The three laureates: Susumu Kitagawa (Middle), Richard Robson (Right), and Omar M. Yaghi (Left). Image from: https://prometheanparticles.co.uk/nobel-prize-recognition/
My organic chemistry professor once asked me, when I consulted him for my future career plan: What is staring in humanity's face right now?
This question has left me pondering, until the 2025 Nobel Prize in Chemistry offers one answer. On the surface level, it tells a story of three chemists and a new class of materials. At a deeper level, it reflects how humanity might re-engineer its relationship with carbon, water and pollution. The Royal Swedish Academy of Sciences awarded the prize to Susumu Kitagawa, Richard Robson and Omar M. Yaghi “for the development of metal–organic frameworks,” or MOFs—crystalline materials that look like ordinary powders but conceal labyrinthine internal architectures. (The Royal Swedish Academy of Sciences, 2025). Within these architectures lie intricate networks of nano-sized pores- capable of capturing gases, filtering pollutants, and storing chemical energy molecule by molecule. The Nobel committee emphasized MOFs’ ability to harvest water from desert air, capture carbon dioxide, store toxic gases, and provide other environmentally critical applications (The Royal Swedish Academy of Sciences, 2025). In an era when climate change, water scarcity, and persistent “forever chemicals” confront humanity, MOFs may be one of the most promising discoveries for resolving these global issues.
To understand the significance of this prize, we must first understand what MOFs are. Chemically, they consist of metal ions or clusters that serve as the “nodes” of a scaffold, connected by organic molecules that act like the struts of the framework. Repeating this pattern in three dimensions, the structure crystallizes into a porous, rigid structure (Geddes & Lee, 2025). Remarkably, a single gram of MOF material can have an internal surface area larger than a football field. Varying the metal centers (such as zinc, zirconium, or copper) and organic linkers (from simple benzene dicarboxylates to complex functional ligands) produces diverse frameworks with different pore sizes, shapes, and chemical properties. This design is known as the reticular chemistry- the practice of constructing matter not molecule by molecule, but as extended networks where the pattern is just as important as the sub-constituent themselves.
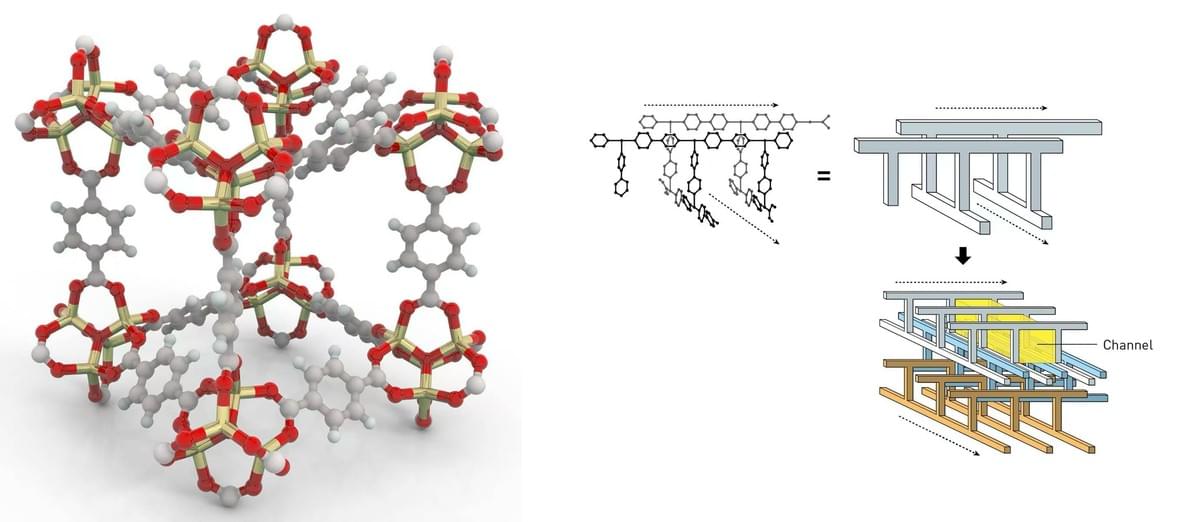
The 3D modeling of MOFs in microscopic view, and potential variability of structures. Image from: https://www.science.org/content/article/architects-molecular-cages-win-chemistry-nobel (Left) https://www.nobelprize.org/prizes/chemistry/2025/press-release/ (Right)
The three laureates each supplied a crucial piece of that network. In the late 1980s, Richard Robson and co-workers showed that it was possible to build infinite three-dimensional coordination frameworks by linking metal centers with rod-like organic ligands, essentially proving that such architectures could exist and could be crystallized. (Kupferschmidt, 2025) Susumu Kitagawa then demonstrated that gases could diffuse into and out of these porous crystals, and that the frameworks could even flex or “breathe” as molecules entered, revealing that they were not just rigid structures but functional sponges. (Nobel Prize) Omar Yaghi advanced the concept into a mature design strategy by creating stable frameworks such as MOF-5, introducing the idea of “secondary building units” as modular nodes, and showing that properties like gas storage and selectivity could be engineered through systematic tuning of the building blocks (Rosi et al., 2003). Together, their contributions completed the puzzle- from the deliberate design to application.
The first level of significance for humanity is that, rather than simply discovering new molecules that naturally exist, chemists are learning to assemble matter in ways that nature rarely explores. This signifies an evolution from the natural scientific way of explaining phenomena to problem solving and engineering. MOFs exactly exemplify this shift. Their pores can be designed to choose one molecule over another, hold onto a gas at one temperature and release it at another, or position catalytic sites in a controlled way along internal channels. (Geddes & Lee, 2025) This is not mere incremental refinement of existing adsorbents like activated carbon or zeolites. It is the articulation of an unprecedented design model that opens a new field of discovery. When the Nobel committee says these materials “contain rooms for chemistry,” they are referring to a platform many different environmental technologies can, in principle, be realized.
Climate change is the most obvious field where those “rooms” might matter. If societies continue to emit gigatons of carbon dioxide each year, stabilizing the climate will have to require both cutting emissions and partially removing CO₂ already in the atmosphere. MOFs are optimal candidates for this task as they can be made highly selective for CO₂ over nitrogen, hydrogen or water vapor due to the presence of adsorption sites in a small volume (Shi et al., 2022). Some frameworks grab CO₂ through chemical interactions with amine groups inside the pores; others rely on tailoring the pore size and electrostatics to create stronger cohesion between CO₂ and the structure. The goal is that such materials could enable carbon capture from power plants, industrial flue gases, or even directly from air. Nevertheless, the most optimistic studies acknowledge that a sorbent, no matter how clever, is only one piece of the puzzle. Carbon capture systems must be coupled to energy-efficient regeneration, long-term storage or utilization of CO₂, and large-scale infrastructure and supportive policy. The Nobel Prize does not change that reality, and it also suggests that one critical component of the technology stack is far more sophisticated than it was a generation ago.
Water scarcity is another domain in which MOFs exhibit significant potential. The Nobel press release highlighted that certain MOFs can condense water from air even in arid desert conditions. (The Royal Swedish Academy of Sciences, 2025) In these systems, the pores are partnered with chemical groups capable of hydrogen bonding to water molecules at low humidity. At night, when the air temperature drops and relative humidity rises, the framework passively adsorbs water vapor. With sunrise, warming releases the captured water, which can then be collected, often with the aid of a simple solar-powered enclosure. Because the water absorption quality of MOFs can be “switched on” at specific humidity thresholds, engineers can select framework tailored to the microclimate of a given region. For a village distant from centralized infrastructure, a compact box of MOF-based sorbent could, in principle, provide a modest but continuous supply of potable water. Yet the distinction between proof-of-concept and societal solution remains critical. The mass of MOF required per liter of water, its stability over thousands of cycles, the cost of production and the durability under dust and heat all determine whether such devices become practical tools or merely remain technological curiosities.
Pollution control and resource recovery form a third front. Many environmental challenges are essentially separation problems: how to remove a trace contaminant from a vast presence of harmless material, or how to selectively recover a valuable component from waste. MOFs are intentionally engineered for this because their pores can be functionalized with groups that bind particular ions, molecules or even complex organic pollutants, while ignoring others (Aquatech Trade News, 2025). Research has demonstrated that MOFs can grab toxic gases, trap volatile organic compounds, and capture emerging contaminants such as pharmaceutical residues or PFAS-like “forever chemicals.” In water treatment, for instance, MOFs might offer faster adsorption, higher capacity, and more tunable selectivity compared to conventional activated carbons. However, scaling laboratory successes into municipal water systems entails confronting a different set of challenges: fouling, regeneration energy costs, regulatory approval, and the uncomfortable question of how to handle spent sorbents now loaded with toxins. A sorbent is not a sink; it is an interim stop in a longer waste-management pathway.
All of this points towards a nuanced answer to whether the the Nobel-winning work can be “the solution” to environmental challenges. In a strict sense, no single material- nor even an entire class of materials- can independently solve climate change, water scarcity, or chemical pollution. These problems are systemic, ingrained in existing energy infrastructure, land use patterns, socioeconomic incentives, and legislations. What MOFs can be is a set of enabling technologies that render certain solutions plausible where they were once impractical. They might lower the energy penalty for carbon capture. They might enable decentralized water supply in places where pipelines or desalination plants are unrealistic. They might let us extract pollutants from industrial streams that current technologies cannot handle practically. In this light, the Nobel Prize recognizes MOFs as a versatile toolset that broadens the design space for the large-scale solutions we will ultimately need.
Future applications of MOF imagined by AI. Image from ChatGPT generation.
The broader scientific ecosystem around MOFs highlights that point. The chemical space of possible frameworks is huge: there are thousands of hypothetical structures, and researchers have turned to machine learning and generative models to explore them. Recent work led by Yaghi and collaborators introduces “agentic AI” systems to propose new MOF compositions, predict their structures, simulate their properties, and even assess their synthetic feasibility before conducting any lab experiments (Shi et al., 2022). In that sense, the 2025 chemistry Nobel intersects with the rise of AI-assisted material science discovery: a feedback loop in which algorithms help human chemists navigate complex designing of material structures, and humans in turn advance algorithms to push more cutting-edge constraints. On a society level, this means that innovation in environmental materials will likely accelerate, and that understanding AI tools is essential for building a robust and equitable product.
There is another layer to the prize that is easy to overlook amid the talk of “supersponges” and futuristic gadgets: the timescale of basic research. Robson’s early coordination frameworks date to the 1980s; Kitagawa’s work on gas adsorption and “breathing” MOFs emerged in the 1990s; Yaghi’s landmark MOF-5 paper is more than two decades old (Kupferschmidt, 2025). Only in recent years have we begun to see serious attempts to deploy MOF-based devices for CO₂ capture, water harvesting, and pollution control (Aquatech Trade News, 2025). The lesson is that today’s grand challenges may be addressed by ideas that begin life as esoteric curiosities in a crystallography lab. Public debates often pit “basic” against “applied” research, but the history of MOFs shows how artificial that divide can be. The same structural elegance that delights chemists in a diffraction pattern may, decades later, underpin a technology capable of keeping a smokestack’s emissions out of the atmosphere.
For humanity, then, the significance of this Nobel Prize lies partly in a kind of disciplined optimism. It would be naïve to suggest that metal–organic frameworks will rescue us from our environmental predicament; emissions must still be reduced, ecosystems protected, and consumption patterns changed. Yet it would be equally misguided to dismiss the role of advanced materials in making such transitions technically and economically feasible. In recognizing MOFs, the Nobel committee affirms a vision of chemistry not merely as a discipline that analyzes the world but as one that builds new platforms on which more sustainable modes of living might be built. For students and young scientists, it signals that work at the interface of molecular design, environmental need, and technology is both intellectually rich and socially consequential.
Ultimately, the 2025 Chemistry Nobel is not a declaration that we have found “the solution” to environmental crises. Rather, it represents the unveiling of a new kind of toolkit- one whose strengths in tunability, porosity, and selectivity align promisingly with some of the hardest separation and capture problems we face. Whether that toolkit becomes central to climate mitigation, clean water access and pollution control will depend on broader choices in energy policy, industrial investment and global equity. The prize reminds us that chemistry can provide powerful instruments, but what those instruments accomplish will be determined less by the precision of their pores than by the wisdom with which we choose to employ them.
Reference
1. The Royal Swedish Academy of Sciences. Press release: Nobel Prize in Chemistry 2025. NobelPrize.org. 8 Oct 2025. https://www.nobelprize.org/prizes/chemistry/2025/press-release/
2. Patel, P. The 2025 chemistry Nobel goes to MOFs. Chemical & Engineering News (ACS), 8 Oct 2025. https://cen.acs.org/people/nobel-prize/The-2025-chemistry-Nobel-goes-to-MOFs/103/web/2025/10
3. Shi, X.; Lee, G. A.; Liu, S.; Kim, D.; Alahmed, A.; Jamal, A.; Wang, L.; Park, A.-H. “Water-stable MOFs and Hydrophobically Encapsulated MOFs for CO₂ Capture from Ambient Air and Wet Flue Gas.” arXiv 2022, arXiv:2211.00787.
4. “A Scientific Investigation, Which Began in 1989 … Has the Potential to Solve Real-World Challenges …” Aquatech Trade News: Nobel Prize for Chemistry Awarded — Three Scientists Developed MOFs with Potential Water Solutions. 27 Oct 2025. https://www.aquatechtrade.com/news/water-treatment/nobel-prize-for-chemistry-awarded-three-scientists-developed-mofs-potential-water-solutions
5. Kupferschmidt, K. 2025 chemistry Nobel prize goes to the scientists behind metal-organic frameworks. Chemistry World 2025, Oct 8. https://www.chemistryworld.com/news/2025-chemistry-nobel-prize-goes-to-the-scientists-behind-metal-organic-frameworks/4022287.article
6. Geddes, H.; Lee, J.-S. “Nobel Prize in Chemistry 2025” Collection. Nature Portfolio. 8 Oct 2025. https://www.nature.com/collections/cjdfehjagc

